R&D
Medica Korea Co., Ltd. established the MK Institute of Regenerative Medicine 'MIRM' in Jiphyeon-dong, Sejong-si in 2019 to focus on renewable medical research and development, and established an efficient development environment such as clean workplaces to study aseptic and biodegradable materials.

Sejong Research Institute
Starting with the development of biocompatible materials, we are preparing medical devices tailored to the needs of the convergence of regenerative materials, and we have established a real-time virus diagnosis system to ensure the safety of materials.
R&D Strategy

Securing new engines through research and development of renewable materials in an aging society
- OECD average life expectancy is 81 years old, joint disease is increasing proportionally with aging
- Diversification of regenerative treatment research using medical collagen
- Applicable to various products such as joint treatment, wound and burn treatment

Product development tailored to needs Technical cooperation through strategic alliance
- Expand and strengthen networks with domestic and foreign pharmaceutical companies, hospitals, animal medical centers, and university research institutes
- Technical cooperation with specialized institutions for regeneration research, product development tailored to needs
R&D Achievement
Secure competitiveness
- Patent : 1 application, 1 registration
| Applied product | Patent name | Application number | Registration number |
|---|---|---|---|
| Atelocollagen | Manufacturing method of high-yield collagen with off-flavor removed | 10-2019-0161751 | 10-2155509 |
| Method for enhancing yield or content of collagen using pH titration | 10-2021-0087970 |
- Atelocollagen virus process validation (International standard)
Signed MOU (5 cases)
| 2022.06 | Signed a business agreement to build a smart factory at Konyang University | |
|---|---|---|
| 2022.03 | Signed an exclusive supply business agreement to expand the raw material market for medical devices at Agathonbio | |
| 2022.01 | Signed a business agreement for pharmaceutical technology-based peptide material development at Incospharm | |
| 2021.08 | Signed a business agreement for medical device development and diagnosis business at Ieum Animal Medical Center | |
| 2019.12 | Signed a business agreement for joint development of material medical devices in Nanding, China |
Assignments (8 cases)
| 1 | Assignment name | 2022 World Class Plus Project - R&D for clinical trial entry and successful commercialization of KDS2010, a non-psychotropic obesity drug |
|---|---|---|
| Department | Ministry of Trade, Industry and Energy(MOTIE) | |
| Date | 2022. 06. 01- 2025. 12. 31 | |
| 2 | Assignment name | Fostering regional specialized industries + (R&D) Fostering regional key industries - Development of high-purity production technology for biodegradable polymer materials for implantable medical devices |
| Department | Ministry of SMEs and Startups(MSS) | |
| Date | 2020. 09. 01 - 2021. 12. 31 | |
| 3 | Assignment name | Biomedical active material base construction project - medical collagen virus confirmation test |
| Department | Intelligent Synthetic Biology Center | |
| Date | 2022. 06 – 2022. 10 | |
| 4 | Assignment name | Biomedical active material base establishment project - Technology consulting for the development of diagnostic kits for degenerative brain diseases |
| Department | Intelligent Synthetic Biology Center | |
| Date | 2022. 06 – 2022. 10 | |
| 5 | Assignment name | Biomedical active material foundation establishment project - Support for licensing of raw materials for health functional food for cognitive function improvement |
| Department | Intelligent Synthetic Biology Center | |
| Date | 2022. 06 – 2022. 10 | |
| 6 | Assignment name | Biomedical active material foundation establishment project - Virus stability test certification for biomaterial medical device filler development |
| Department | Intelligent Synthetic Biology Center | |
| Date | 2021. 05. 01 - 2021. 12. 10 | |
| 7 | Assignment name | Biomedical active material foundation establishment project - Material test analysis |
| Department | Intelligent Synthetic Biology Center | |
| Date | 2020. 10. 01 – 2021. 12. 31 | |
| 8 | Assignment name | Biomedical active material foundation establishment project - Prototype production of aptamer-based jensenoside separation purification column |
| Department | Intelligent Synthetic Biology Center | |
| Date | 2020. 10. 01 – 2021. 12. 31 |
Product
Reagent
Atelocollagen TypeⅠ, from porcine
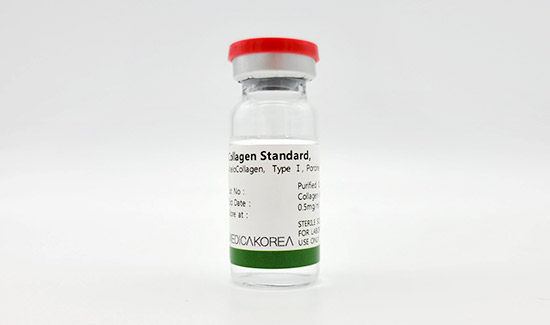
Atelocollagen Reagent 10ml
| Biological source | Porcine skin |
|---|---|
| Form | Solution |
| Type | Atelocollagen type I |
| Content | 0.5mg/ml |
| Storage | 2-8℃ For research use only |
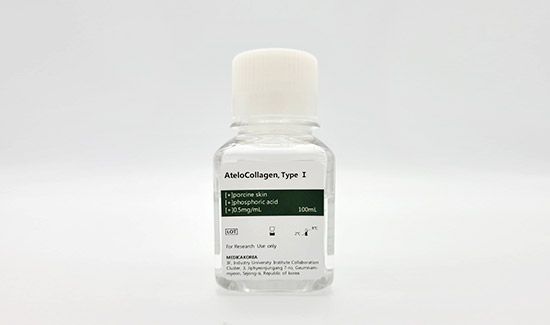
Atelocollagen Reagent 100ml
| Biological source | Porcine skin |
|---|---|
| Form | Liquid |
| Type | Atelocollagen type I |
| Content | 0.5mg/ml |
| Storage | 2-8℃ For research use only |
Raw material
Consultation on specifications such as dosage form, content, quantity, etc. (*Inquire separately)

 KOR
KOR